THE CLINICAL TRIAL
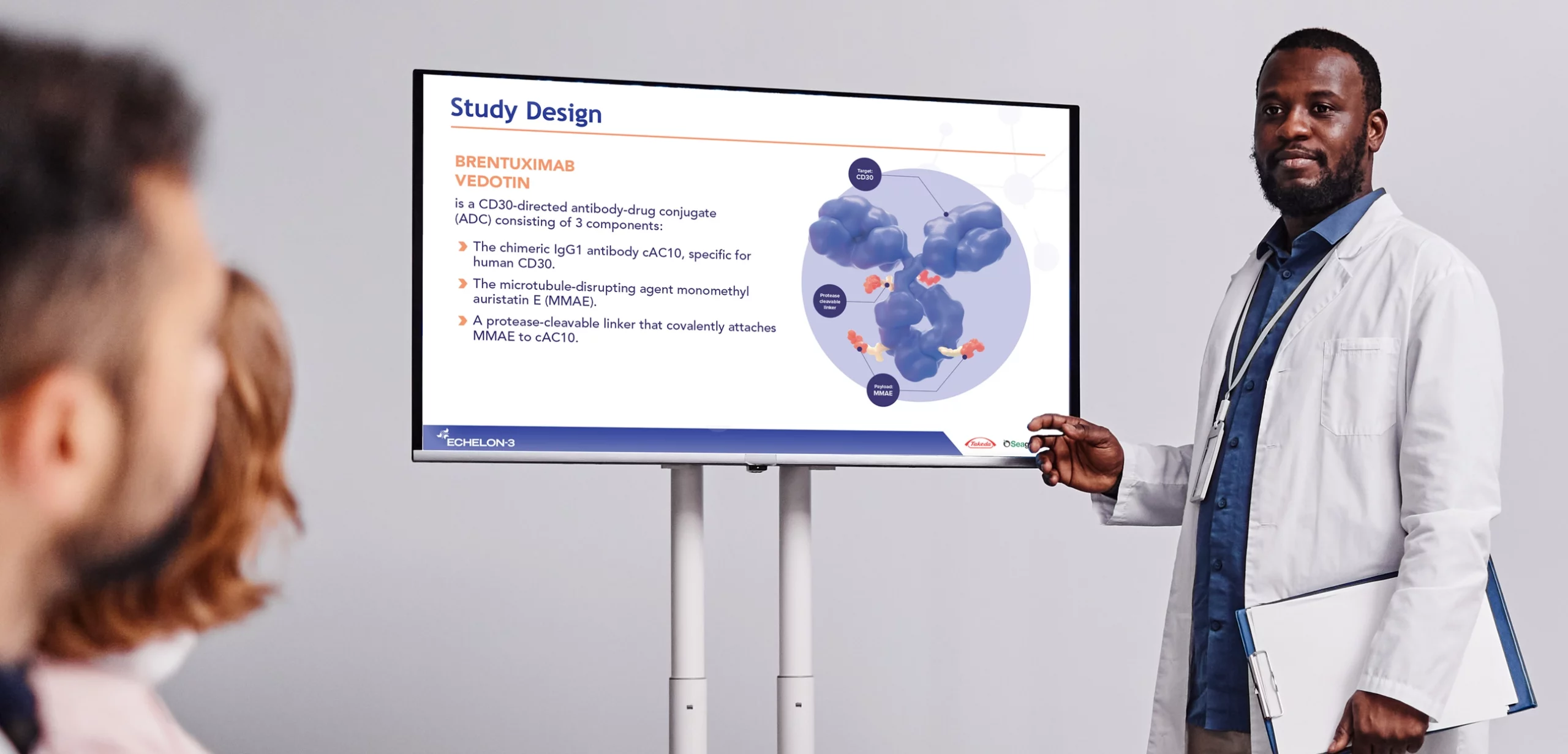
ECHELON-3 was initiated to determine the effectiveness of an investigational drug in treating diffuse large B-cell lymphoma (DLBCL), an aggressive form of Non-Hodgkin’s Lymphoma. Individuals living with DLBCL suffer from the accelerated development of tumors in the lymph nodes, that can spread to their spleen, liver, bone marrow, and other organs. The ECHELON-3 study examined the effectiveness of an investigational drug in combination with standard of care in the treatment of relapsed or refractory DLBCL.
THE PROJECT
For ECHELON-3, the Stark / Raving team was tasked with developing and deploying a robust recruitment toolkit aimed at assisting the Sponsor with enrollment for their study around the world. Cultivating a strong brand identity for ECHELON-3 and synthesizing a highly complex drug method of action into easily digestible language would be key to delivering a complete suite of study materials that resonated with participants and drove engagement with the study staff.