THE CLINICAL TRIAL
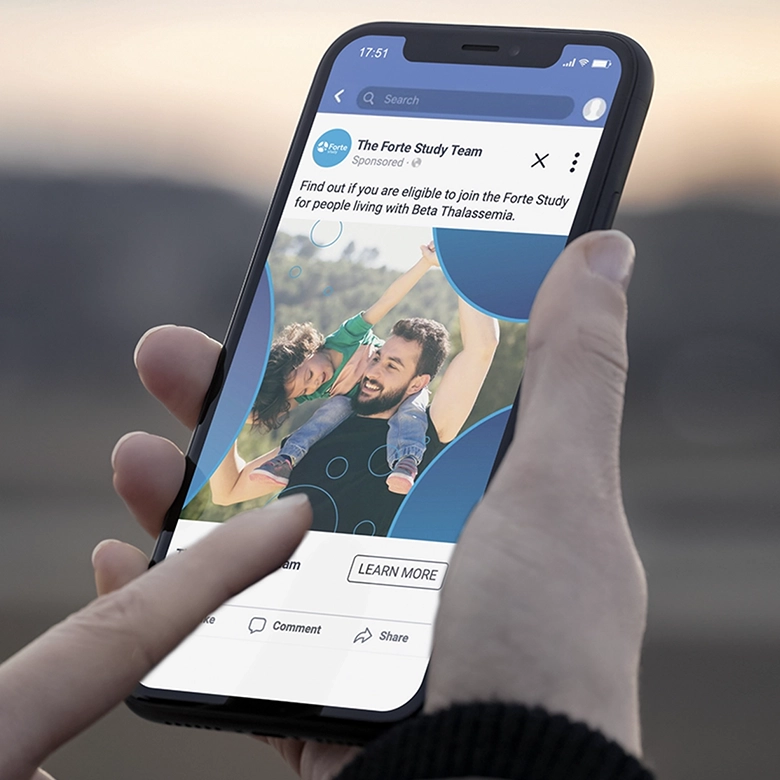
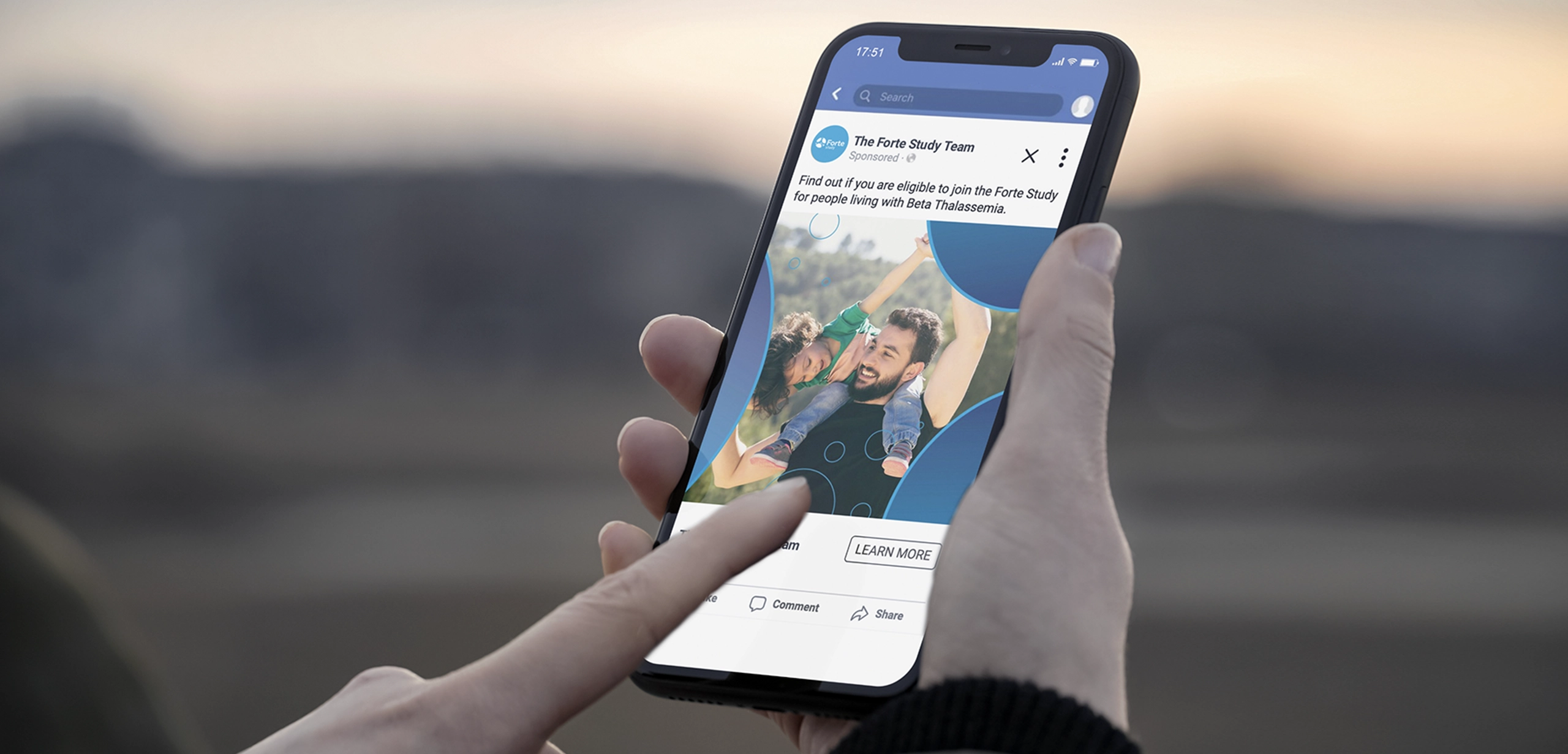
Forte and Ardent were conducted as dual clinical studies that sought to investigate the effectiveness of an investigational drug treating symptoms of Beta Thalassemia and Sickle Cell Disease, respectively. Beta Thalassemia is an inherited blood disorder in which the body does not produce the amount of beta globin that it needs, triggering severe anemia and causing a cascading effect of medical issues. Sickle Cell Disease is an inherited group of blood disorders that impact a person’s red blood cells—causing them to take on a sickle shape—and disrupting the flow of blood throughout the body causing lifelong medical issues. Both conditions significantly impact the quality of life for those who live with them.
THE PROJECT
For the Forte and Ardent studies, the Sponsor needed distinct branding elements to help drive patient recruitment and retention. Stark / Raving jumped in to provide direction on key messaging and creative elements that enhanced the look and feel of the brand, evoking a strong study identity within the site- and patient-facing materials for Forte and Ardent. Utilizing unique design elements, each study stands independently, with consideration given to how the materials would appear once translated and distributed around the globe.